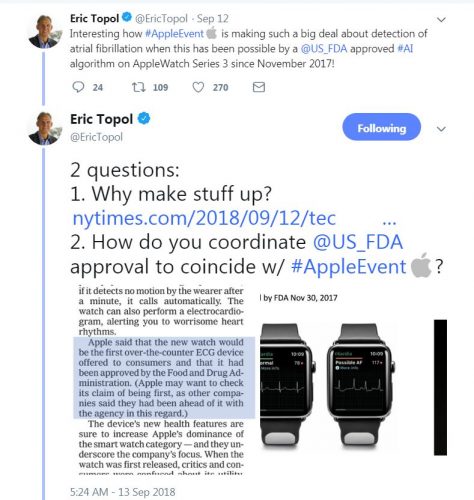
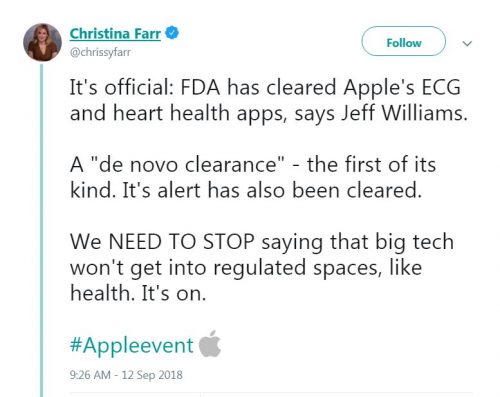
The Apple watch series 4 received FDA approval as a class II medical device. Apple claims that the device can detect falls and arrhythmias. It can also record an electrocardiogram (ECG) in 30 seconds “anytime and anywhere,” according to Apple.
What does it mean to be approved as a class II medical device?
The FDA approved devices fall into three categories: Class I, II, and III. Most devices fall into the class I category which includes the devices that have low to moderate risk to the user such as the bedpans and dental floss. Class II devices have a relatively higher risk to the user compared to class I devices. Examples of class II devices include pregnancy testing kits and powered wheelchairs. Class III devices are the most complex and have the highest risk among the three classes such as implantable cardiovascular defibrillators.
Detecting falls
Falls are one of the leading causes of injuries worldwide. Falls usually have certain patterns like tipping forward or slipping backward. Each pattern of these falls is associated with a certain motion of the body and a movement of the arms. Using its accelerometer and gyroscope, the watch can detect when the user falls.
“These are motions that Series 4 is ideally suited to recognize. With a new accelerometer and gyroscope, the watch analyzes the wrist trajectory and impact acceleration to determine when a fall occurs,” says Jeff Williams, the Chief Operating Officer of Apple.

“This is the first ECG product offered over-the-counter for consumers.” – Jeff Williams
After detecting a fall, the watch delivers an alert and from this alert, the emergency medical services may be called. If the watch detects immobility for more than one minute after the fall, it automatically calls and shares the location with the emergency response team.
Detecting arrhythmias
Apple watches have always had optical heart sensors since their release in 2015. In the previous versions of the watch, it was possible to calculate the resting heart rate and the calorie burnt during the workout via this sensor.
What Apple has introduced this year is the ability to detect bradycardia and notify the user of its occurrence. Not only can the new watch detect rate disturbances, but it also can monitor and detect rhythm abnormalities such as atrial fibrillation.
Atrial fibrillation is the most common arrhythmia worldwide and between 2.7 and 6.1 million Americans have this arrhythmia according to CDC. Williams comments, “The watch won’t catch every instance of atrial fibrillation, but we believe this is going to help a lot of people who did not otherwise know they had an issue”.
Recording ECG
The third new “heart feature” is the ability to record an electrocardiogram. The ECG application -which will come later this year- will be able to record ECG waves as a single lead electrocardiogram does. This new feature is powered by a new electrical sensor in the new watch and is capable of recording an ECG in just 30 seconds, Williams says.
Apple claims that this is the first ECG product offered over-the-counter for consumers. While there were other applications that can record an ECG via the Apple watch such as Kardiaband and AliveCore, this is the first time for an Apple watch to have the app integrated into the Apple watch itself.
Variable feedback from the experts
While some experts have seen this new advance as a sign that technology can get into regulated spaces such as health, others have been skeptical of the benefit of this new technology.
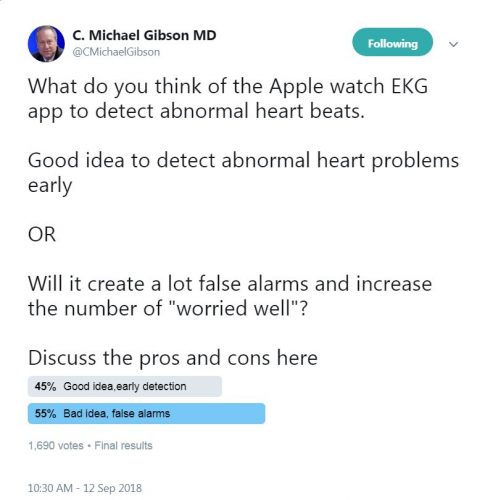
The United States Preventive Services Task Force (USPSTF) has recommended against ECG screening in asymptomatic healthy individuals due to the insufficient evidence that the benefits of this screening outweigh its harm. The concern about the potentially large numbers of false alarms that may be translated into ER visits and serve as an economic burden is another point that is brought up.



Leave a Reply
You must be logged in to post a comment.